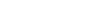Featured Stories, WHOI | March 7, 2013
Inside Quicksilver’s Toxic Transformation
By Genevieve Wanucha
The element mercury, dubbed “quicksilver” by Aristotle, has allowed us to measure temperature and atmospheric pressure, mine precious metals, repair dental cavities, and create energy efficient light bulbs. But coal burning, gold mining, and industrial processes emit thousands of tons into the atmosphere per year, and it all eventually ends up in the ocean. That’s where the trouble starts. Mysterious biological processes tack on a single CH3 methyl group to the metal atom, yielding methylmercury (MeHg), a proven developmental neurotoxin that accumulates in the seafood we eat.
Mercury has extraordinary staying power, cycling in the atmosphere-ocean-land system for up to 3,000 years before returning to the deep ocean sediments. “Once it’s emitted into the atmosphere, it’s like letting a bad genie out of the bottle because you can’t get it back in,” says Michael Bothner, Geochemical Oceanographer Emeritus at USGS Woods Hole Coastal & Marine Science Center.
At constant mercury emissions levels, seawater mercury concentrations in the North Pacific Ocean, a major fishing region, is estimated to double by 2050 relative to 1995 levels, as shown by recent Harvard research. While this scenario grows alarming in the light of the fact that mercury emissions from Asia will increase in the near future, there is huge uncertainty about how much of that mercury will actually convert to its alter ego and get to the seafood counter.
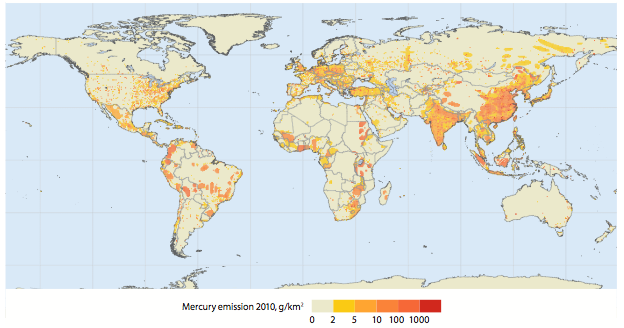
Carl Lamborg, a biogeochemist at Woods Hole Oceanographic Institution, has shown that methylmercury production is not a general feature of the entire ocean but actually occurs in very specific regions. He has established that a low-oxygen layer of water in different parts of the ocean between 100 and 400 meters thick and 100 to 1,000 meters below the sea surface contains high levels of MeHg. Since then, he has grown confident that the MeHg comes from marine bacteria living in that mid-water layer, but they belong to a species he’s yet to track down.
In lakes and sediments, sulfate-reducing bacteria produce MeHg–but not in the ocean. In fact, those bacteria have trouble living in the presence of oxygen, ruling them out as culprits. “It’s frustrating and exciting at the same time learning that what we know about methylmercury in coastal sediments probably doesn’t apply to the ocean,” Lamborg says. “We’ve had to look for whole new explanations.” He and MIT/WHOI Joint Program graduate student Kathleen Munson have found that trace metals may be the answer.
Trace metals naturally present in the ocean, such as zinc, copper, or iron, act as essential micronutrients for marine biota. For example, phytoplankton need iron to survive; the availability of iron in the ocean “limits” the amount of phytoplankton that can grow. Lamborg and Munson recently found evidence that a different trace metal is required by the MeHg producers. “We think that in certain parts of the ocean, the amount of methylmercury being produced is limited by cobalt,” says Lamborg. In other words, it may be that cobalt acts like a fertilizer, enhancing the ability of certain marine bacteria to transform mercury into methylmercury.
Their preliminary finding recently gained new direction from a breakthrough at the Oak Ridge National Laboratory in Tennessee, published last month in Science. There, researchers isolated two genes required for mercury methylation in sulfate-reducing bacteria. Surprisingly, one of those genes encodes a protein that requires a cobalt atom to methylate mercury, which together with Lamborg and Munson’s work, points to the exciting possibility that the ocean’s mystery methylators use that exact cobalt-requiring gene or similar ones. Now, they know what to hunt for. Munson has already collected ocean samples and is just starting to comb through the bits of DNA to determine if those freshly identified genes are more abundant in ocean regions showing methylation.

This work is still far too young to, for example, guide local fishing practices or prevent mercury methylation in the ocean, but understanding mercury methylation is valuable, Lamborg notes, “for learning how this system might respond to changes in total amount of mercury being added to the ocean. If we decide we want to continue burning coal, it would be a good to know how that continued perturbation will manifest itself and if we will see increases in methylmercury in fish or not.”
The United Nations Environment Programme, which agreed in January on a long-awaited global environmental treaty focused on reducing mercury pollution, stated in its own report, the Global Mercury Assessment for 2013: “Under the best-case scenario of maximum feasible reductions, projected estimates for [mercury] deposition in 2050 is similar to estimates for today.” And thus, it’s safe to say the day will never come when tuna and swordfish are safe for everyone. But this MIT/WHOI research hints that somewhere in a complex chain of interactions between mercury, trace metals, and particular genetic profiles of certain marine bacteria, there could be something we can control.